Medior is a global Healthcare company , The endeavor is to export our unique formulations around the globe.
Expanding business and providing affordable healthcare has always been the core philosophy of Medior . Our aim is to ensure better access of affordable quality medicine.
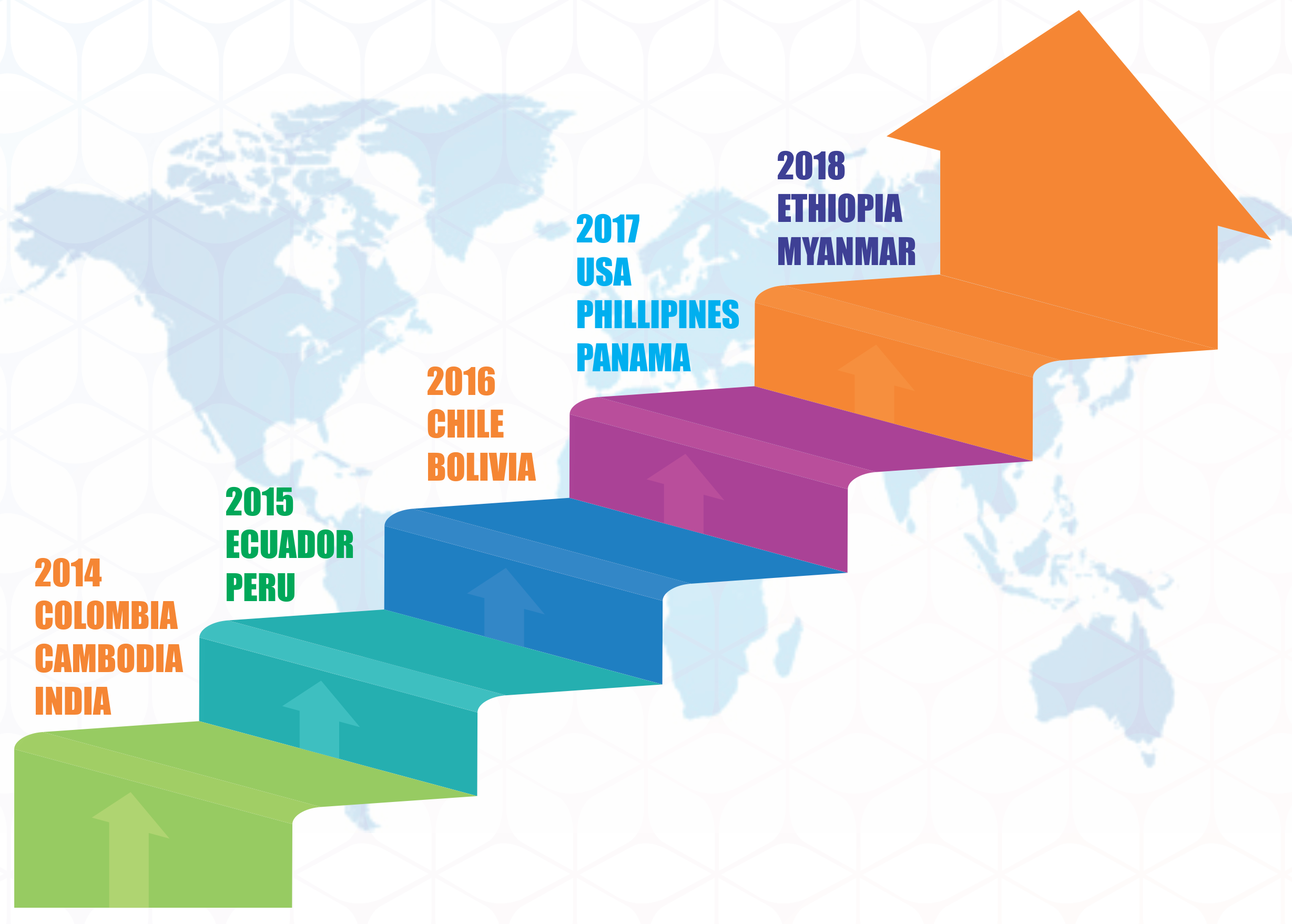
MEDIOR global foot prints in countries like; Vietnam, Cambodia, Sri Lanka, Ethiopia, Philippines, Yemen, Myanmar, Bolivia, Peru, Chile, Uruguay, Ecuador , Colombia Costa Rica Countries etc..
MEDIOR marketing products globally through overseas distribution partner and own subsidiary, over more than 150 registered products. We have filed 300 dossiers in different countries as per CTD, ACTD and guidelines of the MOH of various countries.
MEDIOR is partnering with high esteem and prestige manufactures with full compliance to strict cGMP guidelines and regulated by leading authorities such as WHO, DIGIMED, INVIMA, ARCSA, TGA, US-FDA, UK- MHRA etc...
Medior tireless commitment to quality has earned the trust of its valued customers. Each product passes through a series of stringent checks and controls before being made available to the customer.
We are specializing in providing full sourcing options and expert regulatory support for the widest range of the rarest and newest molecules.
MEDIOR foothold spread to international soil through subsidiary and representation office globally. Medbion Healthcare LLC , USA is pharmaceutical supply chain partner that focus on marketing speciality and OTC products for the US, LATAM and ASIA market.
SERAMED SRL , Bolivia is a subsidiary company of Medior India , one of the fastest growing marketing company to promote locally various dosage forms and OTC range.


DMF ,Dossier (CTD & ACTD) & as per country specific guideline
Preparation / Review /Submission
Product registration: Dossier Submission to various regulatory agencies worldwide
Handling query response and Gap analysis of existing Document
Artwork & Labelling
Ba/BE Studies and assistance for clinical data
GMP /Site due Diligance for API sites , FP sites & Manufacturing sites
Guidance & Assistance for submission of the site/plant registration document to MOH of different country
Pre & Post plant audit and compliance assessment
Development & Preparation of R & D documents
Reference listed Drug (RLD) / Innovator product / Competitor
Market information survey for new molecules